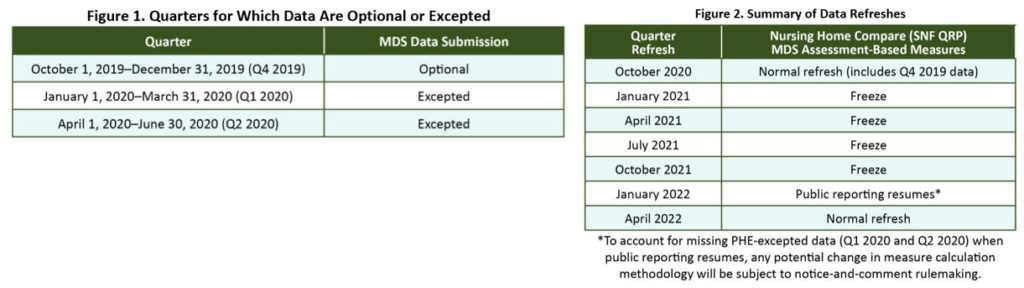
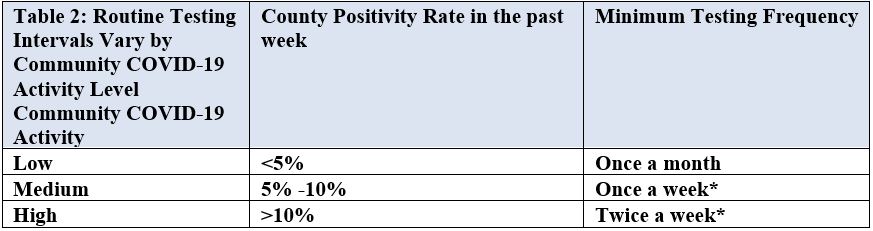
On June 29, 2022, the Centers for Medicare & Medicaid Services (CMS) issued updates to guidance on minimum health and safety standards that Long-Term Care (LTC) facilities must meet to participate in Medicare and Medicaid. CMS also updated and developed new guidance in the State Operations Manual (SOM) to address issues that significantly affect residents of LTC facilities.
Surveyors will begin using this guidance to identify noncompliance on October 24, 2022.
Key areas of guidance include
- Requirements for surveyors to incorporate the use of Payroll Based Journal (PBJ) staffing data for their inspections.
- CMS indicates the believe this will help identify potential noncompliance with CMS’ nursing staff requirements, uncover instance of insufficient staffing, and yield higher quality care. In addition, they state this allows facilities to begin addressing the staffing issues while the new rule making for minimum staffing levels is underway.
- Requirements for an onsite at least part-time Infection Preventionist (IP) who has specialized training to effectively oversee the facilities infection prevention and control program.
- CMS believes that the role of the Infection Preventionist (IP) is critical in the facility’s efforts to mitigate the onset and spread of infections. CMS cites the IP role as critical to mitigating infectious diseases through an effective infection prevention and control program.
- For additional guidance and details, refer to the State Operations Manual and QSO-22-19-NH.
CMS included in memorandum QSO-22-19-NH recommendations related to resident room capacity. There are no new regulations related to resident room capacity. However, CMS wanted to highlight the benefits of reducing the number of residents in each room given the lessons learned during the COVID-19 pandemic for preventing infections and the importance of residents’ rights to privacy and homelike environment. CMS urges providers to consider making changes to their settings to allow for a maximum of double occupancy in each room and encouraging facilities to explore ways to allow for more single occupancy rooms for nursing home residents.
Additional details can be found in the following CMS documents: QSO-22-19-NH, Press Release, Fact Sheet