CMS has released guidance outlining details on how to comply with the new interim final rule requiring COVID-19 testing of staff and residents.
Testing Guidance
CMS is requiring facilities to conduct three types of testing:
- Symptomatic Testing: Test any staff or residents who have signs or symptoms of COVID-19 (facility must continue screening all staff, residents and other visitors).
- Outbreak Testing: Test all staff and residents in response to an outbreak (defined as any single new infection in staff or any nursing home onset infection in a resident) and continue to test all staff and residents that tested negative every 3-7 days until 14 days has passed since the most recent positive result. An admit already confirmed does not constitute a facility outbreak.
- Routine Testing: Test all staff based on the extent of the virus in the community, using CMS’ published county positivity rate under “COVID-19 Testing”, in the prior week as the trigger for staff testing frequency as outlined in the table below:
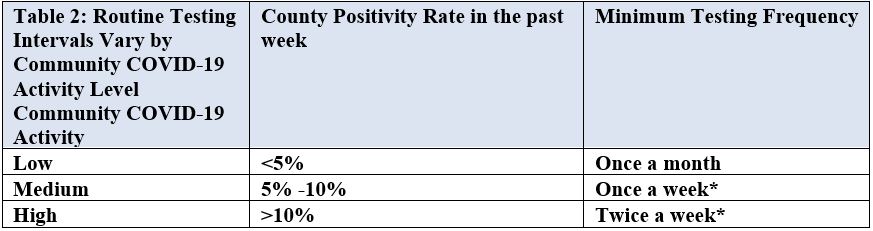
- Facilities are guided to monitor their county positivity rate every other week (e.g. first and third Monday of every month).
- Staff and residents who have recovered from COVID-19 and are asymptomatic do not need to be retested for COVID within 3 months after symptom onset.
- CMS provided guidance on staff who refuse to test:
- Staff who refuse and have signs or symptoms must be prohibited from entering until the return to work criteria are met. CLICK HERE to review the CDC Criteria for Return to Work.
- Asymptomatic staff who refuse testing should follow occupational health and local jurisdiction policies.
- Facilities must maintain records of all testing for compliance and must be able to provide to surveyors.
- Facilities that do not comply with the testing requirements will be cited for noncompliance with new F-tag, F-886.
CMS has also revised the focus surveys for nursing homes to ensure compliance with testing requirements, infection prevention standards, and compliance for infection preventionists.



